
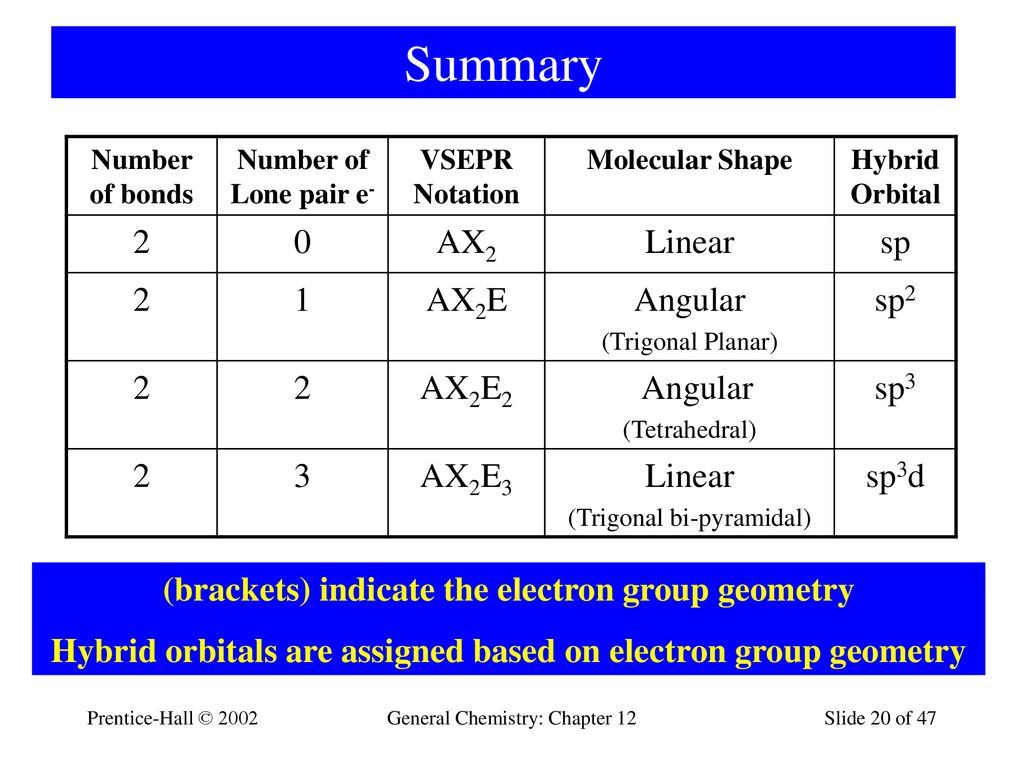
Orbitals are hypothetical structures that can be filled with electrons. trigonal bipyramidal E.Main Difference – sp vs sp 2 vs sp 3 Hybridization NCl3 Question 26 What is the geometry of a carbon atom involved in a triple bond? A. Question 25 Which of the following molecules has three lone pairs of electrons on the central atom? A. CO2 Question 24 Consider the following statements about BCl3 molecules.
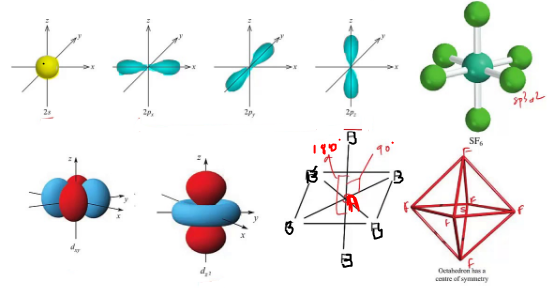
trigonal bipyramidal Question 23 Which of the following molecules is sp3 hybridized at the central atom and has bent molecular geometry? A. tetrahedral Question 22 According to the VESPR Theory, if there are five electron orbitals in the valence shell of an atom they will be arranged in an _ geometry. trigonal bipyramidal Question 21 A molecule whose central atom is sp hybridized will have a _ geometry. 5 and 0 E.8 and 2 Question 20 According to the VSEPR Theory, if there are 3 electron orbitals in the valence shell of an atom they will be arranged in an _ geometry.

it varies Question 19 How many sigma (mc100-1.jpg) bonds and how many pi (mc100-2.jpg) bonds does the ethene molecule contain? A. 5 Question 18 What is the molecular geometry of the species, ICl2+. all except XeF4 Question 17 How many nonbonding pairs of electrons does the central atom Xe have in the molecule XeF4? A. trigonal bipyramidal Question 16 Which choice below is tetrahedral? A. trigonal Question 15 What is the molecular geometry of the PF4+1 ion? A. sp2 Question 14 Predict the molecular geometry of arsine, AsH3. none of these Question 13 A molecule with a trigonal bipyramidal geometry will have a _ orbital hybridization. pyramid Question 12 Which one of the following is a polar molecule with nonpolar bonds? A. ICl3 Question 11 An element that is sp3d hybridized and which has no lone pairs of electrons around it in a molecule is at the center of a(an) _ described by imaginary lines connecting the identical surrounding atoms. Question 10 Which choice below is a square planar geometry? a. Although the electronegativity difference between B and F is large (2.0 units), BF3 is a covalent compound. The B atom does not satisfy the octet rule. BF3 has trigonal pyramidal electronic geometry. BF3 has trigonal planar molecular geometry. 120 degrees Question 9 Which of the following is a false statement about BF3? A. sp3d Question 8 What is the bond angle in the carbonate ion, CO3-2? A.
60 degrees Question 7 What is the orbital hybridization of C in CO2? A. SF4 Question 6 What is the bond angle in the molecule BF3? A. T-shaped Question 5 Which one of the following molecules is polar? A. sp3d2 Question 4 What is the molecular geometry of carbon disulfide, CS2? A. sp3d2 Question 3 What is the hybridization at the central atom (P) of PF3Cl2? A. Which of the following kinds of hybrid orbitals does the cadmium atom likely utilize in CdI2? A. The molecule has the same geometry as a BeCl2 molecule. 60 degrees Question 2 Cadmium iodide, CdI2, is a covalent compound. Question 1 What is the bond angle for the molecule SiCl2F2? A. 60 degrees Question 2Ĭan you answer the following questions please. (Solved) Question 1 What is the bond angle for the molecule SiCl2F2? A.


 0 kommentar(er)
0 kommentar(er)
